Kimberly Raab-Graham, PhD
Professor
Department of Physiology and Pharmacology
Wake Forest School of Medicine
View Biosketch
View Curriculum Vitae
CDMRP Highlight
Science Daily Article
Follow Us On
Instagram and Twitter
Address
|
Where to find us: |
Discover Molecular Mechanisms utilized during Learning and Memory
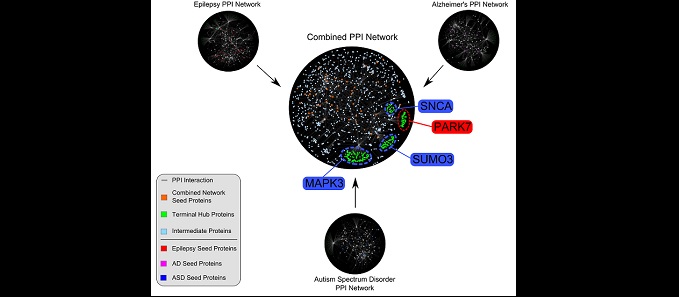
The ability to learn and encode memory relies on changes in proteins that are expressed at or near synapses – sites of communication among brain cells (neurons). My laboratory seeks to understand the biological processes that underlie learning and memory. We are also interested in discovering the molecular pathologies that affect cognition in neurological disorders. We have been studying learning and memory in the context of major depressive disorder (MDD), Alzheimer’s disease (AD), autism spectrum disorder (ASD) and alcohol use disorder (AUD). While these disorders seem different, they share a common problem in the propensity of neurons to have abnormal protein synthesis.
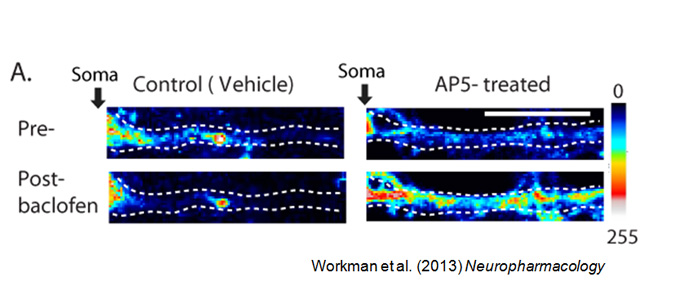
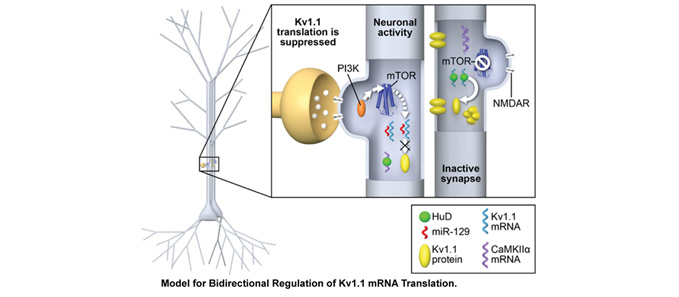
Memory formation requires long-lasting, enhanced synaptic communication (synaptic efficacy). Ongoing studies in the laboratory suggest that synaptic efficacy requires a “yin and yang” approach to protein synthesis. Specific proteins that strengthen communication are synthesized while those that weaken communication undergo translational repression. Often in neuropathological conditions, the balance between translation and repression is disrupted, favoring one state (elevated or reduced protein synthesis) over the other. Our laboratory is actively seeking to identify new drug treatments that restore the balance.
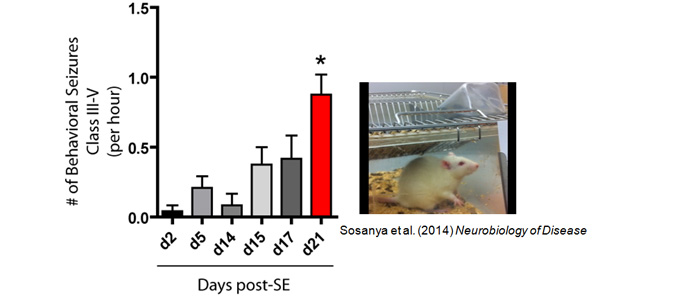
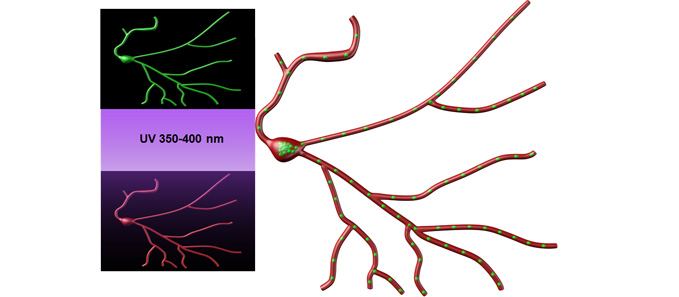
Our experimental approach ranges from molecules to behavior. We use unbiased approaches of mass spectrometry and RNA sequencing, to identify proteins and RNA near synapses. We develop assays to visualize protein synthesis in dendrites. These assays allow us to determine how newly synthesized proteins contribute to site-specific changes in synaptic efficacy. Our findings provide insight into dynamic changes in ion channel expression, function, and behavior across animal models of disease, including depression, epilepsy, autism, and alcohol use disorder. While we have established our molecular understanding of these diseases in mouse models, we plan to extend our studies to include nonhuman primates and humans at Wake Forest School of Medicine.